R&D and prototyping
Deep clinical research, advanced prototyping, and field testing for reliable results.
Mirako Life Sciences partners with innovators and healthcare providers to design, manufacture, and distribute medical grade devices that stand up to real clinical use.
Core device families
Quality system
Market support
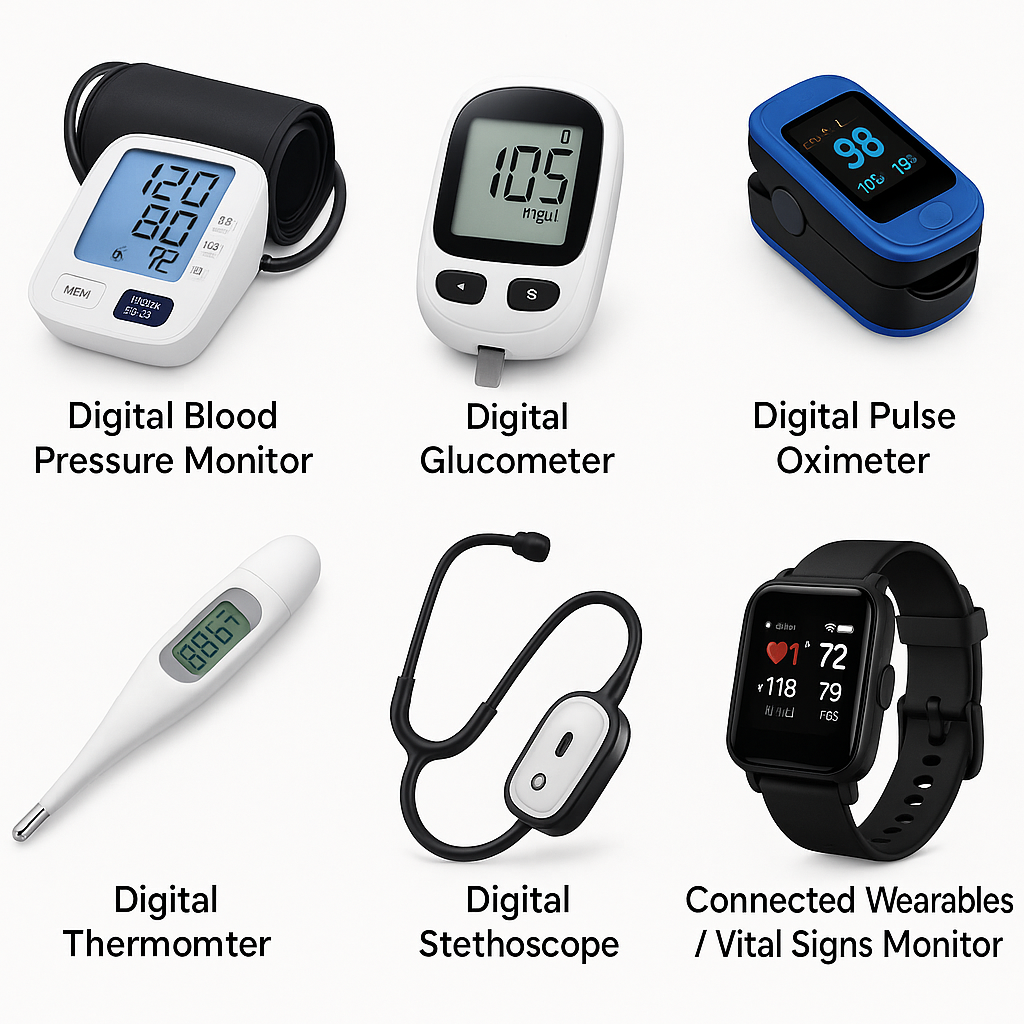
We believe healthcare should be precise, connected, and trusted. Our devices bring clinical accuracy to homes and hospitals.
Deep clinical research, advanced prototyping, and field testing for reliable results.
IoT connectivity and life cycle controls with secure data handling.
Risk management, labeling support, and post market planning.
From sensor to circuit, we verify every material and component that enters our line. Our sourcing partners are audited, our processes validated, and our quality systems built for transparency. What leaves our facility carries the same care it arrived with.
Records tidy, reviews predictable, audits calm. Our integrated ISO 13485 system ensures every process is traceable and compliant.
Maintain clean documentation and continuous design validation with every iteration.
Proactive FMEA and CAPA built into every stage to ensure device reliability.
Transparent qualification and ongoing evaluation of vendors for consistent quality.
Batch-level traceability across design, production, and distribution lines.
Explore Mirako devices and platforms.
Records tidy, reviews predictable, audits calm.

Senior Gynaecologist and Aesthetic Surgeon with four decades of practice, trained in IVF and ART in Mumbai, advanced laparoscopy and endoscopy in Germany and Pittsburgh, and hysteroscopy in Singapore. Introduced regional medical firsts including hyperbaric oxygen therapy. Leads Mirako with scientific precision and compassionate care.

Orthopaedic, joint replacement, and spine surgeon with over 30 years of surgical excellence. Performed more than sixteen thousand total knee replacements and pioneered minimally invasive and robotic techniques. Brings a surgeon’s perspective to device design with reliability and safety.

Engineer and founder who blends computer science and robotics with human centered design. Leads IoT enabled sensors, AI diagnostics, and analytics with a focus on responsibility and reliability.
ISO certified, WHO GMP practices, end-to-end production with calibration, packaging, and traceability.




Tell us what you are building, where you plan to sell, and your target dates.